SPECTROSCOPY
An Introduction to Spectroscopy
Spectroscopy is the study of molecular structure and dynamics through the absorption, reflection, emission and scattering of light (i.e. electromagnetic radiation).
Basics
Atoms and molecules interact with light in two ways:
1) by coupling with the oscillating electric field presented by the wave nature of light, much the way sound can resonate with an object and transfer energy, or
2) as light, interacting with matter in very discrete ways, much the way a moving billiard ball can strike another, stop entirely, and set the other in motion.
These two manifestations of the interaction between light and matter arise from the dual wave-particle nature of light. Therefore, spectroscopy encompasses a broad range of sciences and consequently has many applications.
Applications and Benefits
Spectroscopy is used in a variety of disciplines for identification and quantification of materials. Some key advantages over other analytical methods are that it is non-contact, and is very fast. This is especially important when used in process control applications, such as those in the food industry.
Optical filter applications in spectroscopy utilize the ultraviolet through the infrared range of wavelengths (193 - 16000 nanometers). Within this range, the shorter wavelengths of light interact with the electronic structure of atoms and molecules, and have applications in the biosciences, astronomy and photography. The longer infrared is absorbed at specific wavelengths determined by a molecule's unique structure. These absorption patterns are often used in chemical identification and analysis.
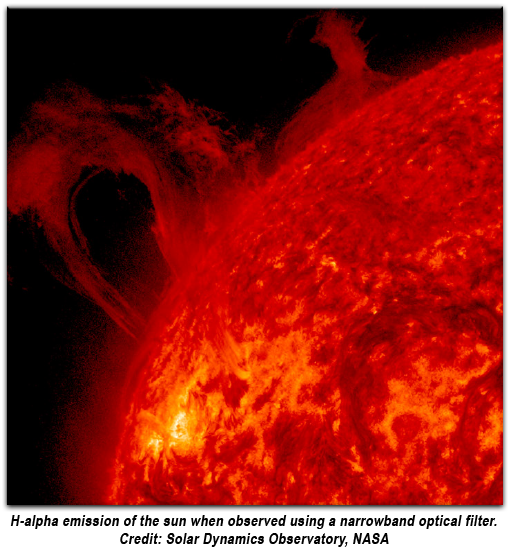
Most sources emit a continuum of wavelengths covering a wide range of the spectrum. Often it is desired to select a specific wavelength to accomplish a task. Optical filters are used to manage the frequency or intensity of light as a function of wavelength. The light can be isolated to specific wavelengths using narrow bandpass filters allow for the transmission of a select range needed to observe discrete electronic transmissions within atoms and molecules. One example would be the isolation of the H-alpha emission of the sun, allowing one to study solar activity in greater detail.
Dichroics and beamsplitters are used to manage broader ranges of wavelengths. An example is the separation of white light into red, green and blue components, allowing for the observation of less discrete transitions within molecules, as in fluorescence or bioluminescence studies. Whereas absorption in the UV and visible are dominated by excitation of electrons, the infrared absorption occurs due to the excitation of molecular vibrations and rotations.
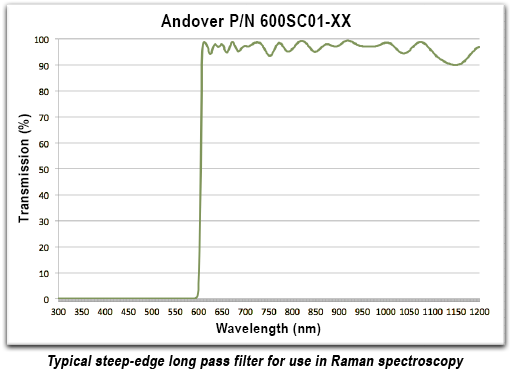
One exception to this is Raman Spectroscopy. The Raman effect results from the absorption and emission of Rayleigh scattered light at wavelengths shifted from the excitation wavelength. The shift results from a molecule's transition into a different vibrational state. Therefore, Raman spectroscopy is a form of infrared vibrational spectroscopy even though the measurements are performed using visible light. Since the emitted wavelengths are so close to the laser excitation wavelength, very steep edge filters are required to block the laser and allow for observation of the weak emission.
Molecules are arrangements of atoms bonded together by the sharing of electrons. The resulting structure is not static but will vibrate because the bonds are flexible, much like a spring, and also the entire molecule can rotate when in the gaseous state. The vibrations and rotations appear as oscillating dipoles and can interact with infrared light to produce adsorption. These absorption frequencies can be used to determine the structure of a molecule.
Molecular infrared absorptions are also used as a monitor for the presence of gases such as Carbon Monoxide/Dioxide, Methane, Water and many others. Infrared bandpass filters will isolate a specific wavelength unique to a molecule such as Carbon Monoxide at 4.67 microns.
Summary
By exploiting the interaction between electromagnetic radiation and matter, spectroscopy enables us to explore, analyze and better understand our physical world.
Authored by Robert Pursel, M.Sc.
Senior Optical Coating Engineer, Andover Corporation
References:
Le Roy, Robert J. "A Spectroscopy Primer" (2003-2011) (http://scienide2.uwaterloo.ca/~rleroy/c209/Primer.pdf)
Baeten, Vincent, and Pierre Dardenne. "Spectroscopy: developments in instrumentation and analysis." Grasas y aceites 53.1 (2002): 45-63.